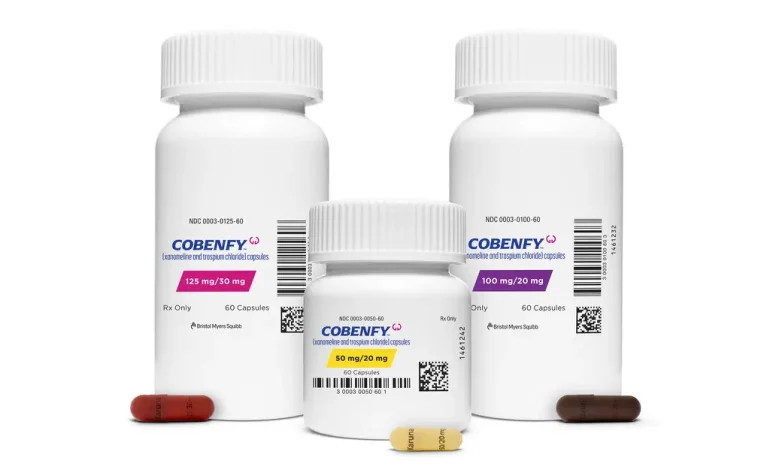
The US Food and Drug Administration (FDA) has approved the first new medication for schizophrenia in over three decades.
Manufactured by Bristol Myers Squibb, the drug, called Cobenfy, combines two active ingredients, xanomeline and trospium chloride, and is administered as a twice-daily pill. The approval marks a significant step forward in treating schizophrenia, a chronic mental health disorder affecting millions worldwide.
Schizophrenia is a severe condition characterized by symptoms such as hallucinations, delusions, and disorganized thinking. Existing antipsychotic treatments primarily target dopamine levels in the brain, which can lead to debilitating side effects like weight gain, drowsiness, and movement disorders. These side effects often result in patients discontinuing their treatment, with some studies showing that 60% to 70% of patients stop taking their medication within 18 months.
Cobenfy offers a new approach, focusing on acetylcholine, another neurotransmitter associated with memory, learning, and attention, rather than dopamine. This novel mechanism of action may help manage schizophrenia symptoms more effectively while reducing common side effects. In clinical trials, Cobenfy demonstrated a lower dropout rate of 6% due to side effects, compared to the 20-30% seen with traditional antipsychotics.
“This drug takes the first new approach to schizophrenia treatment in decades… This approval offers a new alternative to the antipsychotic medications people with schizophrenia have previously been prescribed,” said Dr. Tiffany Farchione, director of the Division of Psychiatry at the FDA.
Despite its promise, Cobenfy is not without side effects. Patients in clinical trials reported issues such as nausea, constipation, and dizziness. However, experts are hopeful that the reduced severity of these side effects will make Cobenfy a viable option for those who have struggled with traditional treatments. Dr. Leslie Citrome, a psychiatry professor at New York Medical College, believes that Cobenfy’s unique mechanism could benefit patients who have not responded well to existing drugs.
With an estimated 24 million people affected by schizophrenia globally, the need for new treatment options is pressing. Schizophrenia can lead to significant challenges in daily life, and if left untreated, it may result in lifelong disability. The approval of Cobenfy provides a new tool for managing this disorder, and additional studies are underway to explore its use in conditions such as Alzheimer’s disease and bipolar disorder.
Cobenfy is expected to be available for prescription by the end of October, offering a fresh avenue of hope for patients and their families.
ABC News, the Washington Post, and the New York Times contributed to this report.








The latest news in your social feeds
Subscribe to our social media platforms to stay tuned