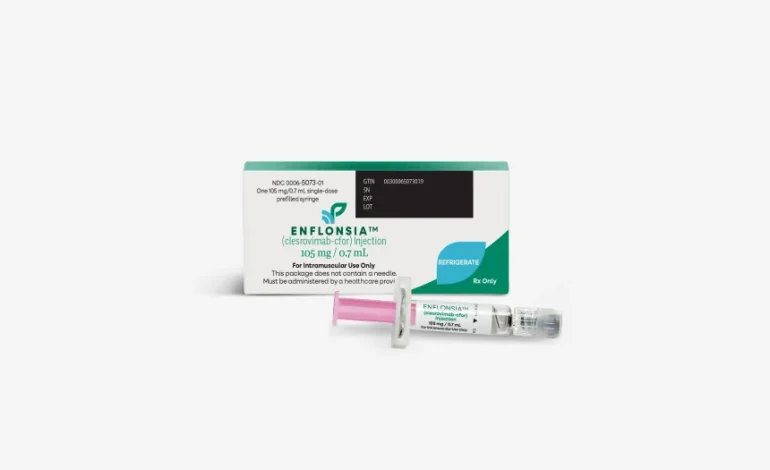
In its first official action, a newly appointed vaccine advisory committee to the US Centers for Disease Control and Prevention (CDC) has voted to recommend the use of Merck’s monoclonal antibody, clesrovimab (brand name Enflonsia), to protect infants from respiratory syncytial virus (RSV).
The shot is now poised to become an additional defense against a virus that hospitalizes tens of thousands of young children in the US each year.
The panel, part of the CDC’s Advisory Committee on Immunization Practices (ACIP), voted 5-2 in favor of recommending the antibody shot for infants under 8 months old entering their first RSV season, particularly those not already protected by maternal vaccination. In a separate, unanimous vote, the committee also approved including the shot in the federal Vaccines for Children Program, which provides free or low-cost vaccines to eligible families.
RSV is one of the leading causes of infant hospitalization in the US, responsible for approximately 58,000 hospitalizations and several hundred deaths among children under five each year. Clesrovimab joins a growing set of tools to combat severe RSV infections, including Beyfortus, another monoclonal antibody from Sanofi and AstraZeneca, and Pfizer’s Abrysvo vaccine, administered during pregnancy.
The ACIP’s vote came after data presented by CDC and Merck experts showed that monoclonal antibodies significantly reduce RSV-related hospitalizations in infants. Dr. Cody Meissner, a pediatrician and committee member, called the findings a “spectacular accomplishment” with major public health implications.
However, the vote also highlighted tensions within the new committee, which was entirely reconstituted earlier this month by US Health and Human Services Secretary Robert F. Kennedy Jr., who dismissed the prior panel of 17 experts over concerns about conflicts of interest. Some public health experts and organizations, including the American Academy of Pediatrics, have since expressed reservations about the scientific direction of the new committee.
Two committee members, including Dr. Retsef Levi of MIT and Dr. Vicky Pebsworth of the National Vaccine Information Center, voted against the recommendation, citing concerns about safety data and trial interpretation. Despite this, both FDA and Merck representatives emphasized the rigorous safety testing behind clesrovimab.
Currently, the final approval of the committee’s recommendation lies with the CDC director—a position temporarily vacant while nominee Dr. Susan Monarez awaits Senate confirmation. Until then, Secretary Kennedy may issue the final sign-off.
In addition to the vote on RSV protection, the committee discussed future reviews of the childhood immunization schedule and longer-term vaccine safety studies. Plans include new working groups to examine the cumulative effects of childhood vaccinations and to reassess immunizations that haven’t been updated in over seven years, including the timing of the hepatitis B vaccine at birth.








The latest news in your social feeds
Subscribe to our social media platforms to stay tuned