With input from the New York Times, the Washington Post, Axios, Politico, Reuters, and the Hill.
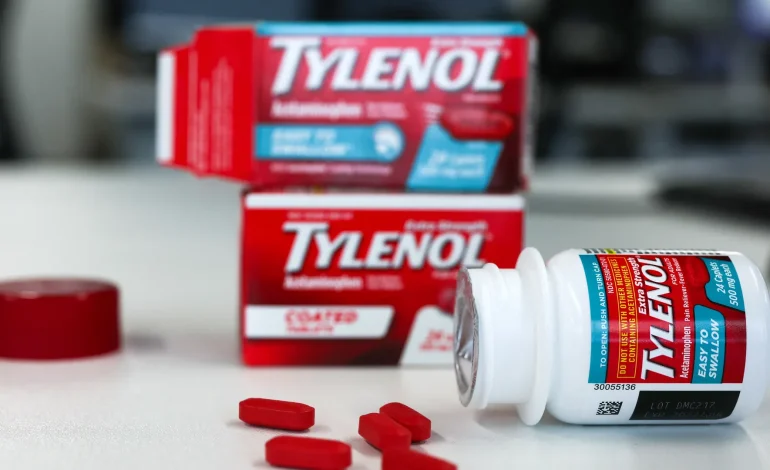
Texas Attorney General Ken Paxton is taking Tylenol to court, accusing Johnson & Johnson and its consumer spinoff Kenvue of hiding risks he says the painkiller poses to babies when taken during pregnancy. The complaint lands a few weeks after President Trump urged pregnant women to avoid acetaminophen, a claim that ricocheted across social media despite the fact that the science doesn’t prove a causal link to autism or ADHD.
Paxton filed the case in Panola County, a rural venue near the Louisiana border that’s often friendlier terrain for conservative litigation. He frames the lawsuit as a consumer-protection action rather than a classic injury case, arguing the companies deceptively marketed Tylenol as safe in pregnancy while concealing evidence of neurodevelopmental harm. He also alleges Johnson & Johnson spun off Kenvue to escape liability tied to the brand, an assertion the complaint offers as a theory more than a documented fact.
The companies are pushing back hard. Kenvue calls the claims baseless and warns that amplifying misinformation about acetaminophen could scare patients away from treating fevers during pregnancy, which doctors agree can be dangerous for both mother and fetus. Johnson & Johnson points out that Kenvue now owns the brand and its liabilities, and in other proceedings the company has emphasized its longstanding warnings about Tylenol’s real, known hazard: liver injury when overdosed. Neither company accepts the notion that they concealed credible evidence of autism risk.
The science that underpins this fight is complicated and, so far, inconclusive. Researchers have spent years probing whether prenatal acetaminophen exposure is associated with autism or ADHD. Some observational studies report a correlation, including a recent review from teams at Harvard and Mount Sinai that summarized dozens of papers and found many showing a statistical link. But it didn’t generate new data, and correlation isn’t causation. Other large studies, including a massive Swedish cohort, suggest that once you account for maternal genetics and other confounders, the association fades. U.S. and European regulators have read the same literature and come to a cautious middle ground: the evidence is mixed and does not prove that Tylenol causes neurodevelopmental disorders.
That regulatory posture is why the Food and Drug Administration’s latest move was nuanced. The agency said it will start the process to update labels to acknowledge a possible association with conditions such as autism and ADHD while stating plainly that a causal relationship hasn’t been established. Kenvue opposes adding such language, arguing it’s not supported by the weight of the evidence. Medical societies representing obstetricians and pediatricians, meanwhile, continue to advise that acetaminophen remains the preferred over-the-counter option for treating pain and fever in pregnancy when used as directed, in part because uncontrolled high fevers carry known risks.
Legally, Paxton is threading a different needle than the private injury suits that have been filed around the country. The biggest federal batch of those cases was tossed last year after a judge found the plaintiffs’ expert testimony unreliable; an appeal is scheduled for November 17. Those lawsuits have to clear the high bar of proving that acetaminophen actually caused a child’s condition. Paxton’s complaint leans instead on Texas consumer law, alleging deception and failure to warn, which lets him argue the companies misled the public even if science hasn’t settled causation. It also revives the narrative that Johnson & Johnson offloaded consumer brands, including Tylenol, into Kenvue to shed legal baggage. The company announced the split in 2021 and completed it in 2023, telling investors it wanted to focus on faster-growing pharmaceutical and device businesses. Wall Street chatter at the time revolved more around talc and opioid exposure than Tylenol, though the acetaminophen litigation was already bubbling.
None of this is happening in a political vacuum. Paxton is challenging Senator John Cornyn in a Republican primary and has built a profile out of headline-grabbing lawsuits that track with Trump-world priorities. Trump himself has continued to post warnings against Tylenol on Truth Social, despite the lack of new evidence and pushback from his own health officials. Advocates aligned with Health and Human Services Secretary Robert F. Kennedy Jr. have petitioned the FDA for stronger acetaminophen warnings, and Kenvue has filed a detailed rebuttal. The courtroom drama has even nicked the market; Kenvue’s stock slumped after Trump’s first salvo and has struggled as headlines multiplied.
For patients and clinicians, the center of gravity remains the same. No court can rewrite clinical guidance overnight, and the medical consensus still holds that acetaminophen is appropriate in pregnancy when a doctor recommends it and the label is followed carefully. The open scientific questions deserve serious research without fear-mongering, and the label debate belongs in a transparent regulatory process, not just a press conference or a lawsuit filing. Paxton’s case will test how far a state can go in punishing companies for not adopting a warning that regulators themselves haven’t endorsed as causal. It will also test whether a political megaphone can bend a nuanced scientific conversation into a blunt legal one.
The stakes are obvious: Tylenol is about as ubiquitous as an American medicine gets. If the Texas suit unlocks internal documents that shift how regulators read the evidence, that could ripple into national labeling and spawn copycat actions by other attorneys general. If the case craters on the same scientific shoals that sank the federal suits, it will reinforce the status quo and maybe cool the political temperature. Until then, the prudent approach for expectant parents is maddeningly unexciting: talk to a clinician, treat fevers, read the label, and ignore the viral posts promising certainty where the science still hasn’t found it.







The latest news in your social feeds
Subscribe to our social media platforms to stay tuned